TAG-NGPM+™
Next Generation Pathogen Media
with virus inactivation and biocide
- A novel virus inactivating transport media – FOR RESEARCH USE ONLY
TAG-NGPM + is a molecular assay specimen collection and transport media with strong virus inactivation and biocide features.. It is designed to stabilize samples in ambient temperature and could serve as an excellent SARS-CoV-2 buffer reagent media for collection and transportation of samples to be used in PCR, rtPCR, recombinase-based isothermal assay (RPA/RAA) , and antigen tests. TAG-NGPM+ is designed to provide better stabilization of RNA sequence and protein structures under more conditions.
Features
- Unique formulation with wide IP protection
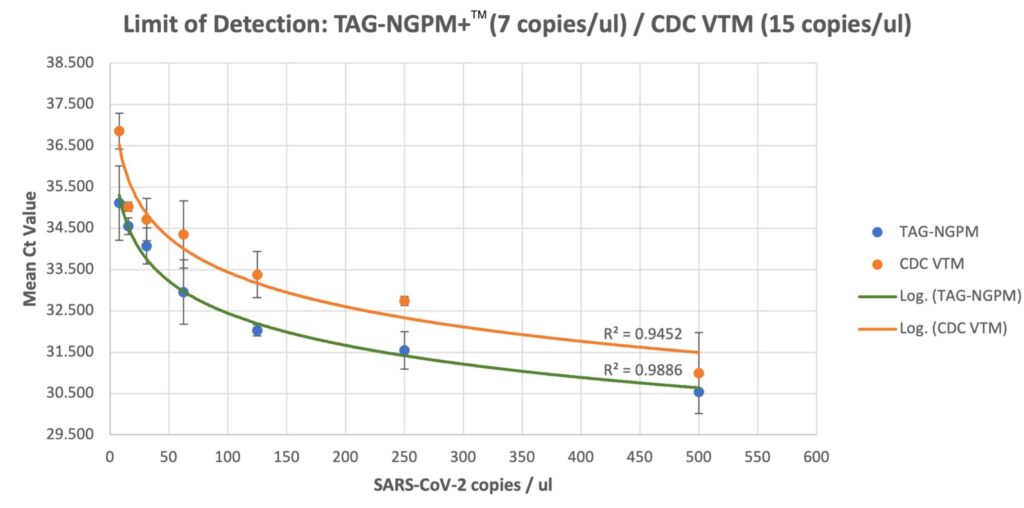
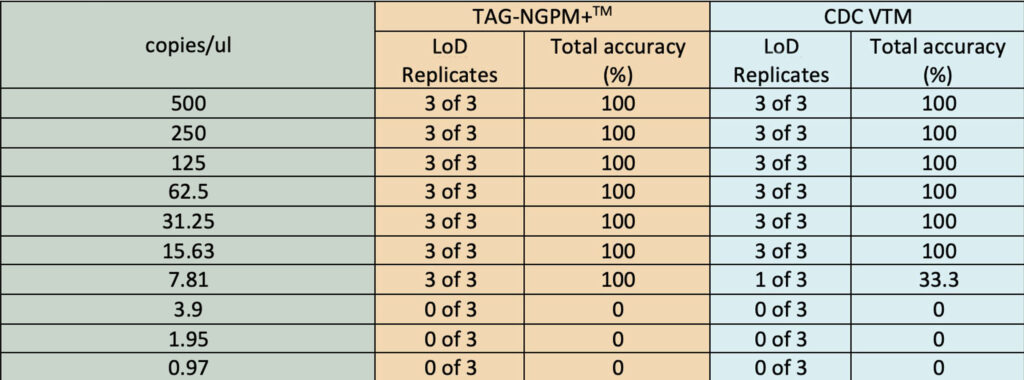
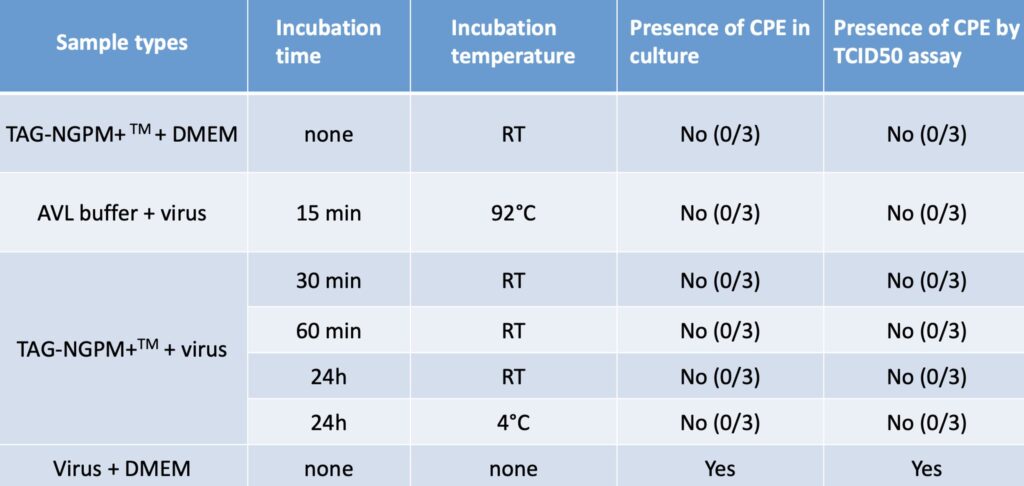
- Strong virus inactivation components. See research results below.
- Available in 1-4ml pre-filled in sample transport tubes.
- Can be kitted with swabs
- Does not form precipitation
- Compatible with EPA approved disinfectant such as sodium hypochlorite solution (NaOCl)
- Contains biocide that prevents microorganism contamination
Benefits
- Temperature stable transport and storage saves significant cost by removing the need for a cold chain. RNA is kept stable at room temperature for 4+ days.
- Pathogen inactivation within 30 minutes of sample collection that reduces risk of exposure
- Better RNA stabilization performance compared to CDC VTM
- Protects RNA from extreme temperature changes such as freeze thaw
- Universal compatibility for downstream assays: RT-qPCR, lateral flow assay, antigen assay, etc.
- Safe to use with standard laboratory decontamination reagents such as bleach
- Inhibits bacteria and fungal contamination
- Compatible with leading platforms including Hologic Panther and Roche Cobas
- Lower price and higher value than any other VTM, UTM and ITMs
- High volume fulfillment available now
- Designed for nasopharyngeal, oropharyngeal, and saliva test kits
- Designed to better preserve RNA fragments and proteins in samples to enable more accurate tests
- Made in the USA
- Validated in Lab Developed and EUA COVID-19 tests. In deployment by test kit providers now