Research
Join us is solving preanalytical variability problems of tissues and pathogens that will increasingly be used in molecular tests..
Improving patient sample stability, quality, and safety for next generation molecular and antigen tests.
Research areas include:
Quantitative measures of infectiousness with low viral load patients. Early detection of infection is critical and requires highly sensitive tests with samples retaining as much viral RNA fragments as possible from collection to processing. Conversely, later stage infections may show positive for RNA fragments long after a patient is no longer infectious. New tests are being developed to solve this issue with including innovation to combine measures of related nuclear proteins together with RNA fragments. Truckee Applied Genomics is supporting research on this topic as it will require better sample transport media for which TAG-NGPM™ and TAG-NGPM™+ are ideally suited.
Green virus inactivating sample media: TAG-NGPM+ is a RUO virus inactivating transport media that is non-flammable, quickly and thoroughly inactivates virus, and contains no guanidine (so can be used on Hologic Panther and other platforms with a bleaching step – and is easier to handle and dispose of). TAG-NGPM comes in a variety of formulations to suite client needs. Some of the forulations contain compenents fororable to EU REACH regulations, yet these show lower levels of virus inactivation. Truckee Appleid Genomics is supporitng related research to better quantify the medical and busienss needs for green reagents with the needs for speed and effectiveness of virus inactivation.
Formalin fixed tissue in molecular pathology
Formalin destroys genetic material.
FFPE is difficult to process and yields inconclusive tests.
As molecular pathology grows, FFPE problems become increasingly costly. Inconclusive tests due to TIFA (Tissue Insufficient For Analysis) are often directly caused by the destructive properties of formalin including sequence fragmentation and low reads on sequence. Workaround kits for processing FFPE require complex, precision processes and add cost.
Truckee Applied Genomics is addressing the problems of FFPE in molecular pathology by researching and building universal non-toxic tissue stabilization reagents that aim to directly replace FFPE with no changes to lab protocols, and that yield better results in molecular pathology without complex processing kits.
Today, Truckee Applied Genomics offers TAG-1, a Class 1 general reagent, substantially equivalent to formalin. Our early research on both new applications for TAG-1 and customized derivative formulations holds great promise. In addition to our research with UC Davis, we are actively seeking research partners to test TAG-1, and work with our company on custom formulations to improve your research outcomes.

2019 TAG-1 Research Results
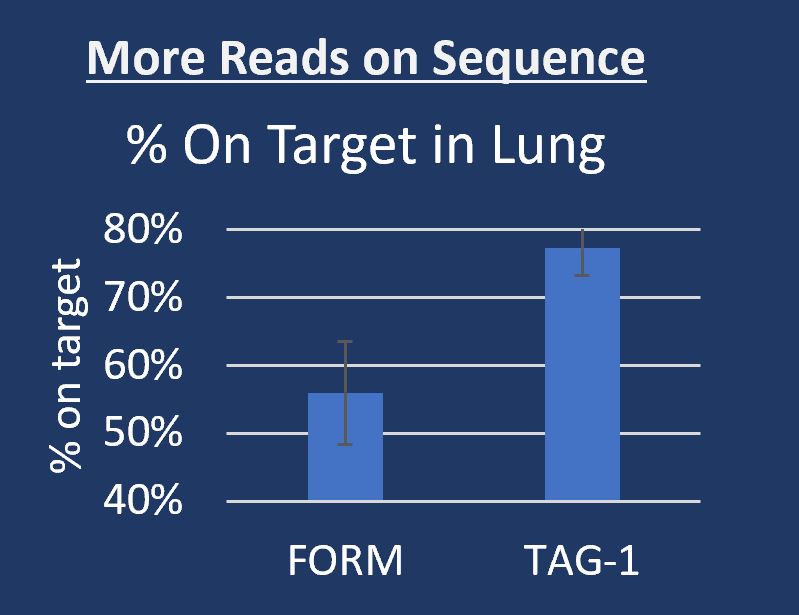
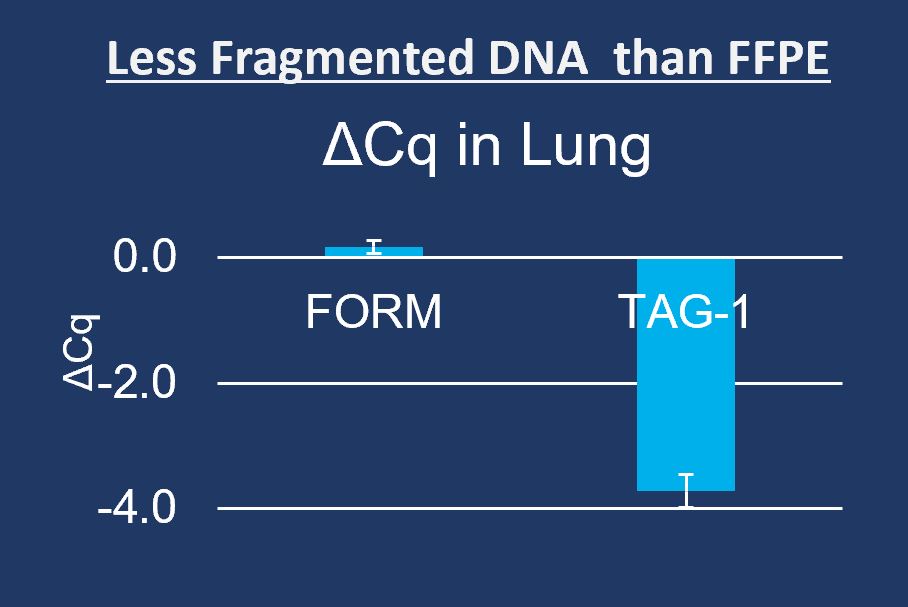
- Identical tissue staining and cell morphology
- Equal to or better than formalin nuclear staining – predictable, just as with formalin.
- No major processing artifacts
- No permeation into the FFPE blocks
- No shrinkage at 30 ml
Researcher Partners

Alexander “Sandy” D. Borowsky, MD
Pathology and Laboratory Medicine – Center for Comparative Medicine
University of California, Davis
ccm.ucdavis.edu/people/alexander-sandy-borowsky
Dr. Borowsky is a Surgical Pathologist with expertise in Diagnostic Breast Pathology. His research training includes characterization of transgenic mouse models of prostate cancer and the molecular analysis of a fusion oncogene central to the pathogenesis of MALT lymphoma.

Padmapriya P. Banada, PHD, MS
Department of Medicine
Rutgers University
https://njms-web.njms.rutgers.edu/profile/myProfile.php?mbmid=banadapp
Dr. Padmapriya Banada obtained her PhD degree from Mysore University, India for her
work on developing molecular methods for the detection of enterotoxigenic
staphylococci and application in food quality programme. She worked as a postdoctoral
research associate at Bhunia lab in Purdue University, on molecular diagnostics,
immunoassays, cytotoxicity assays, lab-on-chip diagnostics and optical light
scattering technology. She joined New Jersey Medical School as a research scientist
to work with Dr. David Alland. Dr. Banada was a key player in the development of
Xpert MTB/RIF assay and part of the team developing the Xpert MTB ultra. She
developed the novel sample processing methods for blood and stool samples for
detection of TB from immunocompromised patients and children. She is now leading
projects on development of advanced diagnostics in bio-defense and sepsis with
current focus on fungal sepsis and hemorrhagic fever viruses, including Ebola virus.
Dr. Banada has more than 15 years of experience in molecular diagnostics of
infectious diseases and tuberculosis. She is a recipient of Purdue Agriculture team
award and has 5 US and European patents/product licenses to her credit. She has
published over 32 peer-reviewed papers, several book chapters and reviews. She worked
in collaboration with FIND and CDRC on several clinical trials in Africa, involving
Xpert MTB/RIF assay for detection of TB in blood and stool.

Jeffrey P. Gregg, MD
Department of Pathology and Laboratory Medicine
University of California, Davis
health.ucdavis.edu/publish/providerbio/cancer/454
Jeffrey P. Gregg’s research laboratory has been dedicated to understanding the genetic basis of cancer using two fundamental biological processes, the genetic predisposition for cancer and molecular alterations (changes in DNA, gene expression and protein) that occur in individual tumors.

John Bishop, MD
Vice Chair for Clinical Services, Department of Pathology and Laboratory Medicine
University of California, Davis
health.ucdavis.edu/publish/providerbio/search/1225
Dr. Bishop’s clinical and research interests include gynecological surgical pathology and cytopathology, including applications of image analysis, molecular and immunohistochemistry, and automated imaging systems.

